How does the desulfurization gypsum process work?
The desulfurization gypsum process is an essential step in reducing the harmful emissions produced by cement plants. So, how does it actually work?
Flue gas containing sulfur dioxide (SO2) is generated during the combustion of fuel in the kiln. This gas is then directed to a desulfurization unit where it comes into contact with limestone slurry. The reaction between SO2 and calcium carbonate present in the limestone slurry forms calcium sulfite.

How does the desulfurization gypsum process work?
Next, this calcium sulfite undergoes oxidation to produce synthetic gypsum. This synthetic gypsum has similar chemical properties as natural gypsum and can be used as a substitute for raw materials in cement production.
The final step involves separating and drying the synthesized gypsum from other impurities before it can be utilized further.
By implementing the desulfurization gypsum process, cement plants are able to reduce their environmental impact by effectively capturing and converting harmful pollutants into useful resources, while also complying with government regulations.
Understanding how the desulfurization gypsum process works allows us to appreciate its significance in promoting sustainable practices within the cement industry.


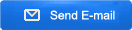
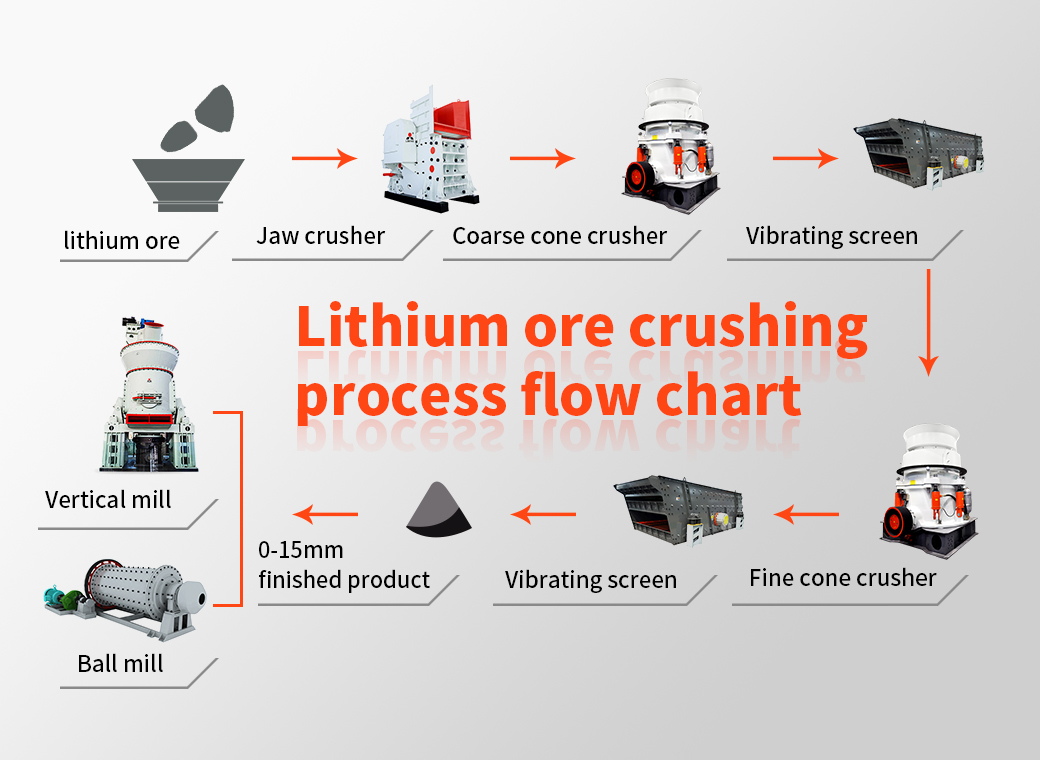
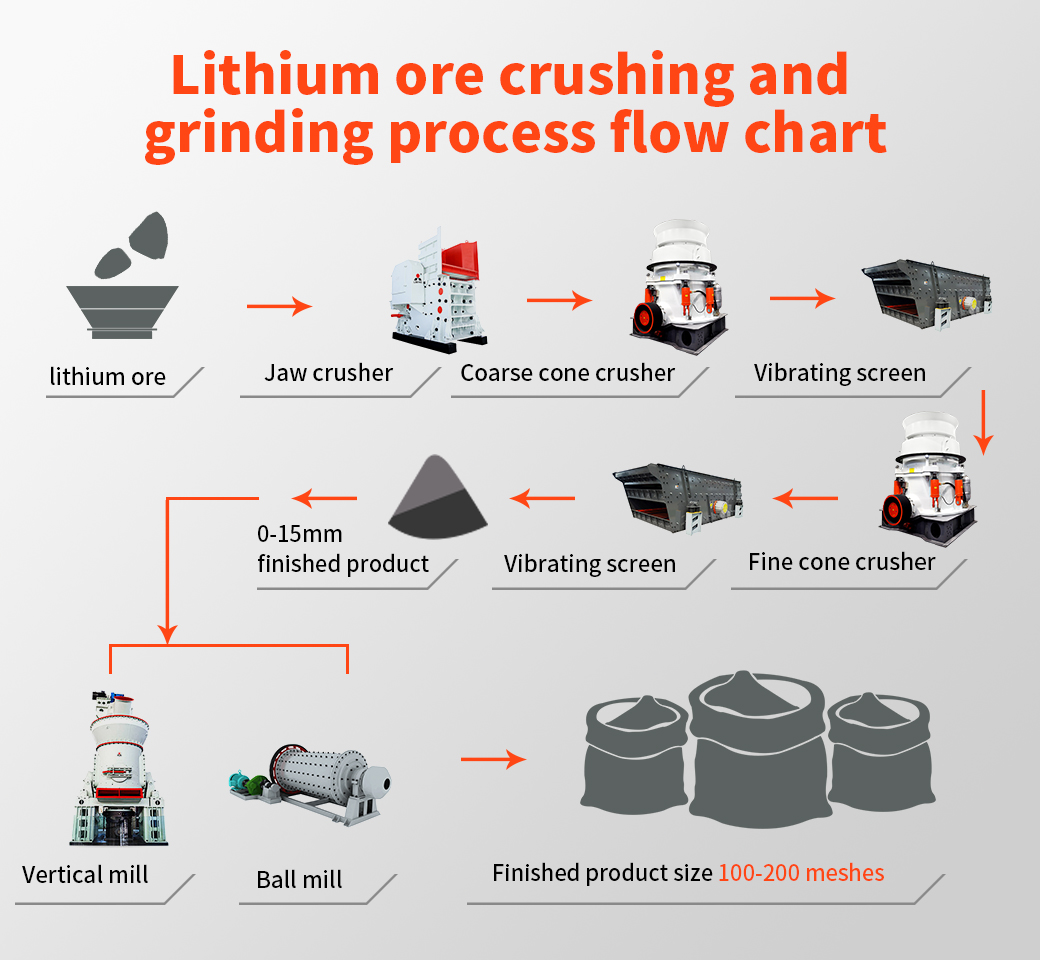 Spodumene: According to the hard rock crushing process, the crushed product is generally 5-40mm, combined with different design requirements of customers, two-end or three-stage crushing, high-grade crushed products (above 4-5%) can be directly used in the metallurgical process to produce lithium carbonate Or lithium hydroxide, the particle size of the finished product is generally around 20-40mm; low-grade generally requires ball mill grinding and separation, and the particle size of the finished product is generally around 5-20mm;
Spodumene: According to the hard rock crushing process, the crushed product is generally 5-40mm, combined with different design requirements of customers, two-end or three-stage crushing, high-grade crushed products (above 4-5%) can be directly used in the metallurgical process to produce lithium carbonate Or lithium hydroxide, the particle size of the finished product is generally around 20-40mm; low-grade generally requires ball mill grinding and separation, and the particle size of the finished product is generally around 5-20mm;